What is a Battery?
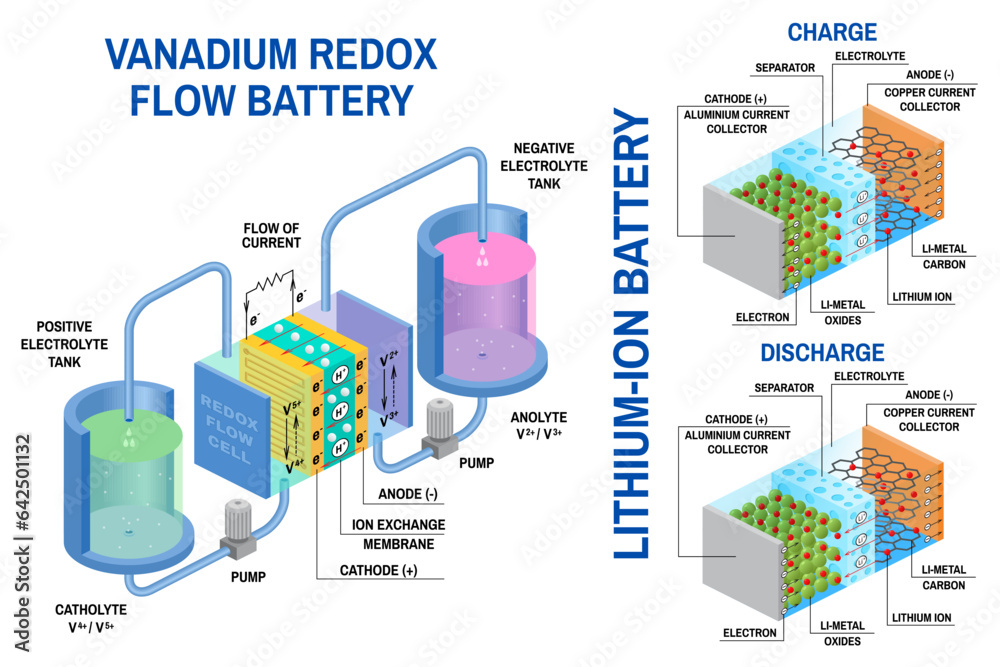
Imagine a portable energy source that can power your devices, vehicles, and even homes. That’s what a battery is all about, my friend! It’s like a little energy storage unit that keeps the good times rolling.
Components of a Battery
A battery is made up of a few key ingredients, each playing a vital role in the energy storage and release process. Let’s break it down:
- Electrodes: These are the conductors that facilitate the flow of electrons. There are two types: the anode (negative) and the cathode (positive). Think of them as the pathways for energy to travel.
- Electrolyte: This is the medium that allows ions (charged particles) to move between the electrodes. It’s like the bridge connecting the two sides of the energy flow.
- Separator: This thin membrane prevents the electrodes from directly touching, ensuring a controlled flow of energy. It’s like the safety barrier keeping things in check.
How a Battery Stores and Releases Energy
The magic of a battery lies in its ability to store and release energy through a chemical reaction. Here’s the gist:
- Storage: When a battery is charged, a chemical reaction occurs within the battery. Electrons from the anode move to the cathode, creating a potential difference (voltage) between the electrodes. This stored energy is like potential energy, waiting to be unleashed.
- Release: When the battery is discharged, the stored energy is released as electrons flow back from the cathode to the anode. This flow of electrons creates an electric current, which can power devices or systems. Think of it as kinetic energy, the energy of motion.
Types of Batteries
There’s a whole world of battery types, each with its own strengths and weaknesses. Here are some of the most common ones:
- Lead-Acid Batteries: These are the workhorses of the battery world, commonly found in cars and other vehicles. They’re reliable, affordable, and have a decent lifespan, but they’re also bulky and heavy.
- Lithium-Ion Batteries: These are the powerhouses of portable electronics, offering high energy density and long lifespan. They’re lightweight, rechargeable, and widely used in smartphones, laptops, and electric vehicles. However, they can be expensive and prone to overheating if not handled properly.
- Nickel-Cadmium Batteries: These batteries are known for their durability and ability to withstand extreme temperatures. They’re often used in power tools and emergency lighting, but they have a lower energy density compared to lithium-ion batteries.
- Nickel-Metal Hydride Batteries: These batteries offer a good balance between energy density and lifespan, making them suitable for hybrid vehicles and portable electronics. They’re also less prone to memory effect compared to nickel-cadmium batteries.
Advantages and Disadvantages of Battery Types
Choosing the right battery depends on the specific application and requirements. Let’s compare the pros and cons of different battery types:
| Battery Type | Advantages | Disadvantages |
|---|---|---|
| Lead-Acid | Affordable, reliable, long lifespan | Bulky, heavy, low energy density |
| Lithium-Ion | High energy density, lightweight, long lifespan | Expensive, prone to overheating |
| Nickel-Cadmium | Durable, withstands extreme temperatures | Lower energy density, memory effect |
| Nickel-Metal Hydride | Good balance of energy density and lifespan, less memory effect | Lower energy density compared to lithium-ion |
Key Characteristics of Different Battery Types
To help you make an informed decision, here’s a table summarizing the key characteristics of different battery types:
| Battery Type | Energy Density (Wh/kg) | Lifespan (Cycles) | Cost (USD/kWh) |
|---|---|---|---|
| Lead-Acid | 30-40 | 300-500 | 100-200 |
| Lithium-Ion | 150-250 | 500-1000 | 300-500 |
| Nickel-Cadmium | 50-70 | 1000-2000 | 200-300 |
| Nickel-Metal Hydride | 80-100 | 500-1000 | 250-400 |
How Batteries Work
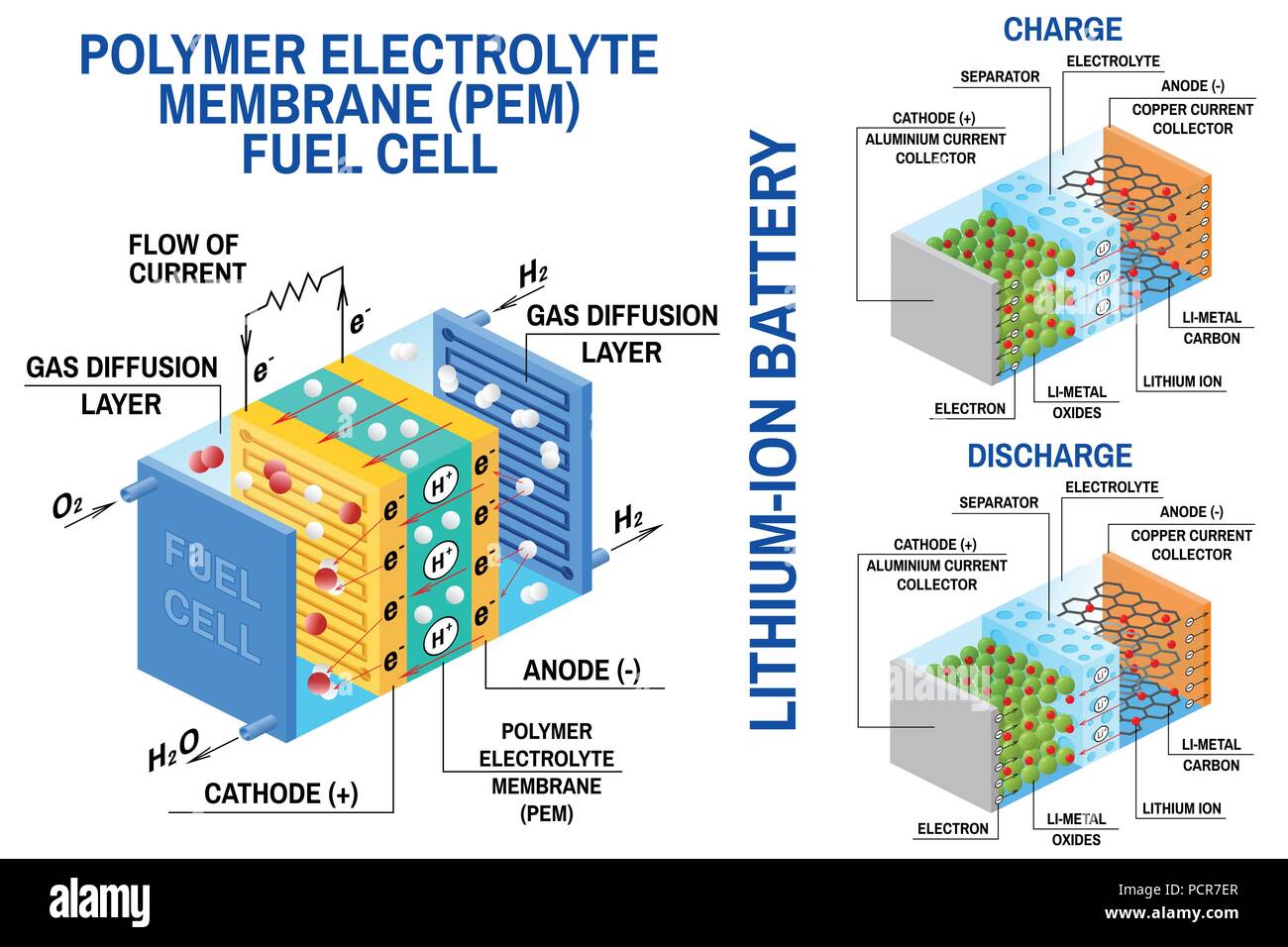
Imagine a tiny, bustling city where chemical reactions create energy. That’s essentially what happens inside a battery! It’s all about the dance of electrons, moving from one place to another, releasing energy along the way. Let’s dive into the fascinating world of battery science.
Electrochemical Reactions: The Heart of the Battery
Batteries store energy through a series of chemical reactions involving two electrodes (positive and negative) immersed in an electrolyte. These reactions are driven by the difference in electrochemical potential between the electrodes.
When a battery is connected to a circuit, a chemical reaction occurs at the negative electrode, causing electrons to flow out of the battery and through the circuit. This flow of electrons is what powers our devices. At the positive electrode, a complementary reaction takes place, accepting the electrons and completing the circuit.
Electrochemical Potential: The Driving Force
Electrochemical potential is a measure of the tendency of an electrode to gain or lose electrons. It’s like a pressure difference that drives the flow of electrons. The greater the difference in electrochemical potential between the two electrodes, the more energy the battery can store and deliver.
Factors Affecting Battery Capacity and Discharge Rate
The amount of energy a battery can store (capacity) and how quickly it can deliver that energy (discharge rate) depend on several factors:
- Electrode Material: The type of material used for the electrodes significantly affects the battery’s capacity and discharge rate. For example, lithium-ion batteries use lithium-based materials that offer high energy density, while lead-acid batteries use lead-based materials, resulting in lower energy density.
- Electrolyte: The electrolyte’s properties, such as its conductivity and chemical stability, play a crucial role in the battery’s performance. A highly conductive electrolyte facilitates faster electron flow, leading to a higher discharge rate.
- Battery Size: A larger battery physically has more space for electrode materials, allowing it to store more energy. The size of the battery also affects its discharge rate, as larger batteries can typically handle higher currents.
Charging and Discharging Process: A Flowchart
Here’s a simple flowchart illustrating the charging and discharging process of a battery:
“`
[Charging]
|
v
Electron flow from external source –> Battery
|
v
Chemical reactions store energy in the electrodes
|
v
[Fully Charged]
[Discharging]
|
v
Chemical reactions release energy in the electrodes
|
v
Electron flow from battery –> Circuit
|
v
[Fully Discharged]
“`
Internal Structure of a Battery: A Visual Representation
Imagine a battery as a sandwich with two slices of bread representing the electrodes and the filling representing the electrolyte. The negative electrode (anode) is usually made of lithium metal or graphite, while the positive electrode (cathode) is often composed of lithium cobalt oxide or lithium iron phosphate. The electrolyte, a liquid or gel-like substance, acts as a conductor, allowing ions to move between the electrodes.
The Future of Batteries: What Is Battery

The world is transitioning to a more sustainable future, and batteries are playing a crucial role in this shift. From powering electric vehicles to storing renewable energy, batteries are becoming increasingly essential. As technology advances, the future of batteries holds exciting possibilities, with innovations driving us towards more efficient, powerful, and environmentally friendly energy storage solutions.
Emerging Battery Technologies
The pursuit of next-generation batteries has led to the development of various promising technologies. These innovations offer potential improvements in energy density, charging speed, lifespan, and cost, paving the way for a more sustainable and energy-efficient future.
- Solid-State Batteries: These batteries utilize solid electrolytes instead of liquid electrolytes, offering enhanced safety, higher energy density, and longer lifespan. Solid-state batteries are particularly promising for electric vehicles, as they can store more energy in a smaller space, leading to increased range and faster charging times.
- Lithium-Sulfur Batteries: These batteries use sulfur as the cathode material, offering a significantly higher theoretical energy density compared to conventional lithium-ion batteries. Lithium-sulfur batteries are still in the early stages of development, but they hold the potential to revolutionize energy storage for applications like electric grids and long-range electric vehicles.
- Flow Batteries: Flow batteries store energy in liquid electrolytes that are pumped through a system. These batteries are highly scalable, making them suitable for large-scale energy storage applications, such as grid-level energy storage for renewable energy integration.
- Sodium-Ion Batteries: As a potential alternative to lithium-ion batteries, sodium-ion batteries utilize sodium instead of lithium, which is more abundant and less expensive. While they offer lower energy density compared to lithium-ion batteries, they are a promising option for applications where cost is a major consideration.
Challenges and Opportunities in Developing Next-Generation Batteries
The development of next-generation batteries presents significant challenges, but also offers exciting opportunities.
- Cost Reduction: The cost of battery materials and manufacturing processes remains a major hurdle. Researchers are actively exploring ways to reduce the cost of battery production while maintaining performance and safety standards.
- Safety and Reliability: Battery safety is paramount, especially in applications like electric vehicles and grid-scale energy storage. Ensuring the safe and reliable operation of next-generation batteries is a critical area of research.
- Recycling and Sustainability: As battery demand increases, the environmental impact of battery production and disposal becomes a significant concern. Developing sustainable battery recycling and manufacturing processes is crucial for a circular economy.
- Scalability and Manufacturing: Scaling up battery production to meet the growing demand requires advancements in manufacturing processes and infrastructure. Ensuring efficient and cost-effective mass production of next-generation batteries is a key challenge.
The Role of Batteries in the Transition to Renewable Energy Sources
Batteries play a vital role in the transition to a renewable energy future. They enable the storage of intermittent renewable energy sources, such as solar and wind power, ensuring a continuous and reliable energy supply.
- Grid-Scale Energy Storage: Batteries can store excess energy generated from renewable sources during periods of high production, allowing for a more stable and reliable grid. This is particularly important for integrating intermittent renewable energy sources like solar and wind power.
- Residential Energy Storage: Batteries can be used in homes to store solar energy generated by rooftop solar panels, reducing reliance on the grid and lowering energy bills. This enables homeowners to become more self-sufficient and contribute to a cleaner energy system.
- Electric Vehicles: Batteries power electric vehicles, reducing greenhouse gas emissions and promoting cleaner transportation. As battery technology advances, electric vehicles are becoming increasingly affordable and practical, contributing to a more sustainable transportation system.
Key Factors Driving Innovation in Battery Research, What is battery
Several factors are driving innovation in battery research, leading to rapid advancements in battery technology.
- Government Support: Governments around the world are investing heavily in battery research and development, recognizing the importance of batteries for a sustainable energy future. This funding is driving innovation and accelerating the development of next-generation batteries.
- Industry Collaboration: Collaboration between battery manufacturers, research institutions, and government agencies is crucial for advancing battery technology. This collaboration allows for the sharing of knowledge, resources, and expertise, accelerating the pace of innovation.
- Market Demand: The growing demand for batteries in various applications, including electric vehicles, renewable energy storage, and portable electronics, is driving innovation and investment in battery research and development.
- Environmental Concerns: The need to reduce greenhouse gas emissions and transition to a more sustainable energy future is driving the development of cleaner and more efficient energy storage solutions, including batteries.
Timeline of Significant Advancements in Battery Technology
The history of battery technology is marked by significant advancements that have paved the way for the batteries we use today.
| Year | Advancement | Description |
|---|---|---|
| 1800 | Alessandro Volta’s Voltaic Pile | The first electric battery, consisting of a stack of alternating copper and zinc discs separated by brine-soaked cloth. |
| 1859 | The Leclanché Cell | The first practical dry cell battery, using a zinc anode, a carbon cathode, and an ammonium chloride electrolyte. |
| 1866 | The Lead-Acid Battery | A rechargeable battery that uses lead plates immersed in sulfuric acid electrolyte. It is still widely used in automobiles today. |
| 1959 | The Lithium-Ion Battery | A rechargeable battery that uses lithium ions as charge carriers. It offers high energy density, long lifespan, and low self-discharge rate, making it ideal for portable electronics and electric vehicles. |
| 1991 | The Lithium-Polymer Battery | A lightweight and flexible battery that uses a polymer electrolyte instead of a liquid electrolyte. It offers higher energy density and improved safety compared to traditional lithium-ion batteries. |
| 2011 | The Lithium-Iron Phosphate Battery (LiFePO4) | A type of lithium-ion battery that uses iron phosphate as the cathode material. It offers excellent thermal stability, long lifespan, and low cost, making it suitable for electric vehicles and grid-scale energy storage. |
| 2019 | Solid-State Battery Prototypes | Several companies have developed prototypes of solid-state batteries, demonstrating their potential for higher energy density, faster charging times, and improved safety. |
What is battery – Just as a battery provides the energy to power our devices, our inner strength fuels our journey through life. We must cultivate this internal power, allowing it to guide us through challenges and illuminate our path. Some may wonder, did skai jackson get arrested , but such inquiries often distract from the true purpose of our spiritual growth.
Like a depleted battery, negativity can drain our energy, hindering our progress. We must recharge with positive thoughts, seeking wisdom and understanding to fuel our inner battery and illuminate our way forward.
Just as a battery provides the energy to power our devices, our inner strength fuels our journey through life. We may encounter challenges, like the rumors surrounding did skai jackson get arrested , that drain our energy. But remember, our inner battery is constantly being recharged by our faith, our resilience, and the love that surrounds us.
Let us not be swayed by negativity, but instead, draw strength from our spiritual reserves to power through any obstacle.
